FDA May Reclassify ECT Devices
Price: $ 168.50
5(196)
The FDA may reclassify electroconvulsive therapy devices from class III to class II. The FDA is accepting public comments on the change until March 28.

The Dangerous Truth About Electroshock (ECT) - Mental Health

When to consider electroconvulsive therapy (ECT) - Kellner - 2020

Advocacy Update: February 2019
Electroshock Therapy (ECT) Trial – Jury Finds Somatics Failed to
FDA Looks to Expand Electroshock Use Despite Significant Risks and

Electroconvulsive Therapy

FDA May Reclassify ECT Devices
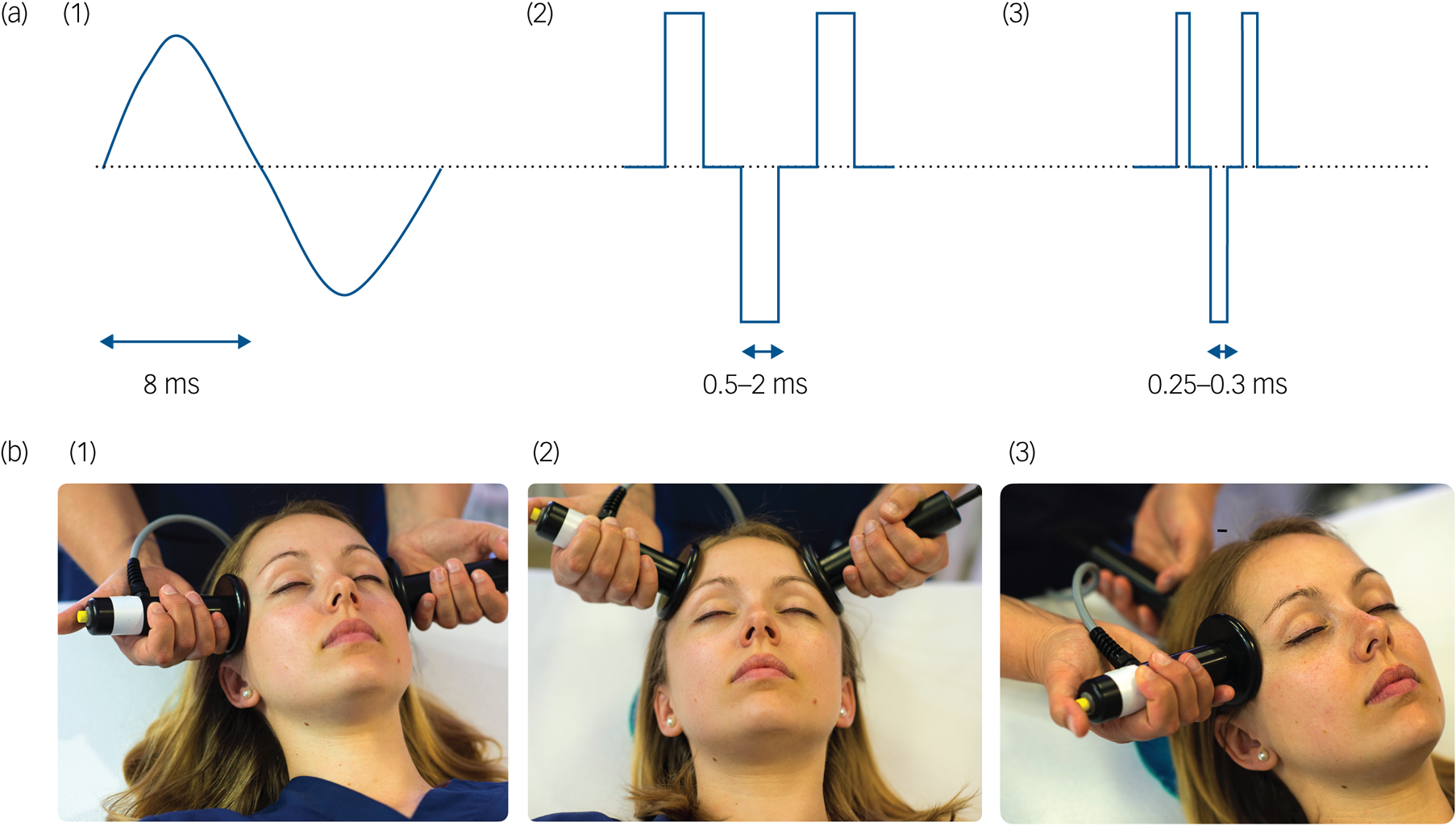
Electroconvulsive therapy for depression: 80 years of progress

FORM S-1/A

How to Report Your ECT Injury to the FDA - Life After ECT
You may also like




