FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood Pressure Monitor
Price: $ 82.99
4.7(627)
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.

FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood

FDA Clears Edwards Lifesciences' Advanced Noninvasive Hemodynamic
Nanowear's SimpleSense-BP secures US FDA 510(k) clearance

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous

Ep 22 Introducing Nanotechnology to improve patient outcomes

Nanowear wins FDA nod for wearable blood pressure monitor
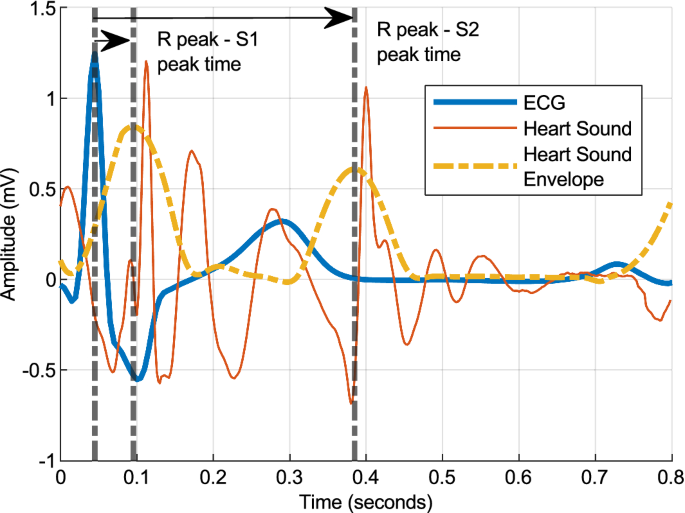
Multiparametric cloth-based wearable, SimpleSense, estimates blood

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous

Non-Invasive Blood Pressure Monitor - Conduct Science

AdvaMed (@AdvaMedUpdate) / X
:max_bytes(150000):strip_icc()/faw-glass-food-storage-containers-test-pyrex-simply-18-piece-set-ashley-schaubroeck-02-230441a0b9744a0684912cfcf5e87dec.jpeg)